|
||||
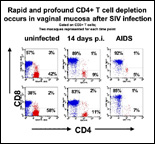
The Division of Immunology conducts basic and applied immunology studies of disease processes with a focus on infectious diseases such as AIDS, Lyme disease and Rickettsia. The Division consists of eleven members including two faculty members, two postdoctoral fellows, six research technicians, and one administrative staff. Dr. Marcelo J. Kuroda, Associate Professor of Microbiology and Immunology at Tulane Medical School, is Chairman of the Division. The functions of the Division can be divided into three main areas: 1) Research, 2) Research Resources and 3) Education and Training Opportunities. RESEARCH PROGRAMSThe research component of the Division of Immunology is active and involves collaborative and independent work in a number of areas focusing on mechanisms of disease and development of new animal models. A major current focus is on basic aspects of cellular immunology in the monkey model of AIDS. We study virus-specific cellular immune responses specific for the AIDS virus in a nonhuman primate model of AIDS, the simian immunodeficiency virus-infected rhesus monkey. These studies are done using both functional assays and flow cytometry, employing tetrameric MHC class I or II/peptide complexes. These tetramers are used as staining reagents in flow cytometric analyses to quantitate and define the phenotype of antigen-specific T lymphocytes. Using these reagents, we were able to demonstrate a clear correlation between cytotoxic T cell (CTL) expansion and clearance of viral replication during primary SIV infection. Moreover, the distribution of virus-specific CD8+ T cells in various lymphoid compartments and their association with localized virus replication has also been assessed. The precise quantitative power of this technology has allowed us to compare different vaccination modalities in order to develop an effective vaccine against HIV infection. We have also succeeded in developing tetrameric MHC class II peptide complexes to quantitate antigen-specific CD4+ T cells in virus-infected and vaccinated rhesus monkeys. We have selected the rhesus MHC class II, Mamu-DR*W201 for the construction of the class II tetramers, because we found that this molecule is expressed in 35-40% of all rhesus monkeys tested from four different colonies. This new technology has facilitated the quantitative analysis of simian immunodeficiency virus (SIV)-specific T cell responses in vivo in monkeys following virus infection and immunization. To better understand the biology of the antigen–specific CD8+ and CD4+ T lymphocytes in SIV infection, we are currently extending the usage of the tetramer technology to other new MHC class I and II alleles. Recently, to expand our ability to analyze the MHC-TCR interaction from the opposite perspective, we have constructed a TCR tetramer by reassembling a and b chains derived from a peptide-specific T cell population into a functional antigen-specific TCR. The TCR tetramer recognized the restricting MHC class I molecule only in the peptide-bound state and with high specificity and avidity. Importantly, subtle changes in cognate peptide levels bound to the class I molecule were accurately reflected by parallel changes in the mean fluorescence intensity of cells that bound the TCR tetramer. Furthermore, we showed that the TCR tetramer blocks expansion in vitro of CTL specific for the cognate peptide, but has no effect on the expansion of CTL specific for control peptides restricted by the same class I allele. The stringency of this tetramer for its restricting class I allele was also demonstrated by accurately identifying animals expressing the restricting Mamu-A*01 class I allele from a large cohort of over 100 outbred rhesus macaques. With its high throughput potential and reproducibility, TCR tetramer-based approaches can help explore the MHC/peptide interface in detail. With the described tools in hand, we are currently using both mouse and rhesus macaque models to study the fate of memory as well as naïve antigen specific CD8+ and CD4+ T cells using dendritic cells as professional antigen presenting cells. Detailed knowledge of the interaction of APC with antigen specific T cells will be needed to design effective vaccine strategies as well as immune-based therapy to infectious diseases, tumors and autoimmune diseases. RESEARCH RESOURCESThe Division of Immunology has a significant service commitment to the Center. These service functions can be divided into two major cores: 1) Flow cytometry core, 2) Immunology core. Flow Cytometry Core (FCC) The FCC is equipped with two FACS-Calibur analytic flow cytometers capable of 4-color analysis, one FACS Aria high-speed cell sorter capable of 9-color analysis and simultaneous 4-way cell sorting and a LSRII analytic flow cytometer capable of 12-color analysis. The FCC offers assistance ranging from study design through data acquisition and analysis. Immunology Core (IM) The IC provides immunology services to specific research projects of in-house and outside investigators, as requested. Current services include sample preparation under standardized procedures for optimal assay analysis, planning and performance of ELISPOT assays, data processing and presentation, and intracellular cytokine staining. EDUCATION & TRAINING OPPORTUNITIES (send email) The Division offers training opportunities to postdoctoral fellows, undergraduate students, graduate students and research fellows in the area of AIDS immunology and vaccine development STAFF (View Publications) Chair – Kuroda, Marcelo J. MD, PhD, Associate Professor of Microbiology and Immunology Faculty Kuroda, Marcelo J. MD, PhD, Associate Professor |
|
©2013 Tulane University |