 |
||||
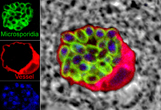
The Division of Microbiology conducts basic infectious disease research using nonhuman primate models of human disease with an emphasis on AIDS, microsporidial infections, vaccine development and retrovirology. The Division consists of thirty-two members including seven faculty, four post-doctoral fellows, four visiting scientists, four administrative staff, ten research technicians and two graduate students. Dr. Preston A. Marx, Professor of Tropical Medicine at Tulane School of Public Health and Tropical Medicine, is Chairman of the Division. The functions of the Division can be divided into three main areas: 1) Research, 2) Research Resources and 3) Education and Training Opportunities. RESEARCH PROGRAMSThe research activities of the Division can be divided into AIDS related and non-AIDS related. Research on AIDS includes the origins and genetic diversity of HIV, pathogenesis as it is related to progression, long term latency and cell biology, a nonhuman primate model for heterosexual transmission of HIV and vaccine development. Non-AIDS research can be grouped into projects involving, microsporidia and its associated diseases, human and simian T cell leukemia virus, varicella virus, respiratory syncitial virus, human and rhesus papilloma virus, and rotavirus. Brief descriptions of major research programs are provided below.Origins and Genetic Diversity of HIV and the Emergence of Pandemic HIVResearch in the Division has focused on identifying SIV genetic lineages that are ancestral to HIV with the goal of understanding the genetic diversity of primate lentiviruses. Scientists in the Division have discovered new SIVs in western and equatorial Africa that are members of the HIV family tree. The ancestral viruses to HIV-1 and HIV-2 are SIV from the chimpanzee and the sooty mangabey, respectively. However, the SIV group infects over 40 monkey species in Africa. One focus is finding out if the other SIVs that infect other African monkey and ape species pose a risk for human infection and the emergence of new HIV strains and types. Another major effort is to understand the steps leading to the transition of SIV to HIV. Pathogenesis of SIVThe SIV animal model is being used to conduct basic research on the immunopathogenesis of SIV infections. The research investigates cross species transmission of SIV, long term non progression and the absence of disease in African nonhuman primates naturally infected with SIV. It is now clear that AIDS originated from cross species transmission of SIV from one or more species of African nonhuman primates to humans. Thus, a better understanding of cross species transmission, emergence, and adaptation of SIV to new human and nonhuman primate hosts is of major importance. The mechanisms by which SIV successfully adapts to new hosts will shed light on the origins of pandemic strains of HIV and will apply to other viruses as well. To address this question, SIV strains obtained directly from sooty mangabeys are being used to infect rhesus macaques to understand how SIV adapts to a new host species. The results of these studies will contribute to the understanding of how new epidemic strains of HIV evolved and emerged in the 20th century. It is well known that HIV infection of humans is fatal in five to ten years if left untreated. However, a small percentage of HIV infected persons suppress the infection naturally and live for longer periods without symptoms of AIDS. Little is known about these elite long-term nonprogressors (LTNP), and it is difficult to study in humans, especially in the early stages of infection. Research in the Division has recently shown that a significant percentage of SIVmac-infected rhesus of Chinese origin become LTNP analgous to elite LTNP in humans. Research is ongoing to understand the dynamics of immune cell restoration and control of infection in these animals. Several species of nonhuman primates native to Africa are naturally infected with SIV and rarely develop disease. Understanding how these animals can maintain a life-long infection without resulting in disease is likely to offer important clues to help develop strategies to prevent AIDS in humans. Toward this end, we are examining African green monkeys and sooty mangabeys. Each species is infected with its own strain of SIV, and each seems to have developed unique mechanisms to cope with the infection. Only two examples of AIDS are known in naturally infected sooty mangabeys and African green monkeys. Both studies were done in the TNPRC Division of Microbiology. Heterosexual Models for HIV TransmissionThe majority of HIV infections occur by heterosexual transmission. Investigators in the Division were the first to develop an animal model to study sexual transmission. Using SIVmac and female rhesus macaques, infection was readily transmitted across the intact vaginal lining. Infection became systemic and resulted in the development of AIDS. This model closely follows AIDS development in HIV-infected women and is being used to study prevention, treatment and pathogenesis. Recent studies in collaboration with the Division of Comparative Pathology have focused on monoclonal antibodies to prevent SIV vaginal infection. There is a critical need for inexpensive drugs that can prevent HIV transmission to women because vaccines are not yet ready for use. Drugs that can be used safely and discreetly by women to prevent HIV infection are of great interest, particularly in developing countries. The monoclonal antibody b12 had been used as a topical prophylactic in the vagina and shown to be effective at blocking infection. However, b12 is rare among antibodies in that it can neutralize HIV and SHIV (a simian human hybrid virus). In ongoing studies, mutants of b12 are being tested to understand the mechanism by which b12 antibody can prevent vaginal SHIV infection. Other uses of the model are the study of the role of the female hormones, progesterone and estrogen, in SIV vaginal transmission. A long-term study has shown that progesterone increases the susceptibility of the vaginal lining to SIV infection. In contrast, estrogen prevents infection. Prevention correlated with an increase in thickness of the vaginal lining which is hypothesized to block infection. This finding can be exploited to develop new prevention strategies. The most recent development applies a vaginal cream containing estrial. Estrogen-based vaginal creams are routinely used by women to treat post-menopausal vaginal symptoms. Estrogen creams induce changes in the vaginal microenvironment that increase resistance to SIV infection. The results have been promising, and a new study is planned in women to examine the changes in the vagina that may promote resistance to HIV infection. AIDS vaccinesAIDS vaccines are a major focus in the Division. Live vectors and immunogenic peptides have been used. The SIV macaque model is also used by Division collaborators having AIDS vaccine programs funded by NIH. Live vaccine vectors have included vesicular stomatitis virus (VSV). VSV is relatively non-pathogenic in monkeys and humans. VSV-HIV hybrid viruses have been constructed to express HIV and SIV genes. The safety, immunogenicity and efficacy of these vectors expressing SIV genes have been tested in the SIV/macaque model of AIDS. Animals have been immunized by intramuscular, oral and intranasal routes to determine which induces the strongest and most durable immune response. The humoral and cell-mediated immune responses were measured. The appearance of antibodies was measured and compared between different VSV-hybrids and different routes of immunization. SHIV was used to challenge immunized macaque monkeys. The candidate vaccine induced strong protection in the SHIV model. The immunized monkeys were not observed to have any deleterious effects from the immunization protocol with the live vectors. Upon challenge with a pathogenic SHIV virus, the immunized monkeys suppressed the virus compared to the mock vaccine control group. Because of the success of this series of experiments,VSV-HIV hybrid vectors are being developed for clinical trials in humans. These new vectors for human use are currently being tested in rhesus macaques to determine if they are safe and immunogenic. Another vaccine approach in the Division is the use of synthetic peptides derived from immunogenic epitopes of the gag and env gene of HIV and SIV. These peptides were incorporated into immunostimulating complexes known as ISCOMS. This and other novel approaches are being pursued in the Division. Natural History of Microsporidial Infections and Therapeutic ApproachesMicrosporidia are single-celled parasites associated with diarrhea and systemic disease in animals and humans worldwide. Horizontal, zoonotic, waterborne, and food borne modes of transmission occur, thereby resulting in targeted research on the microsporidia by the U.S. Environmental Protection Agency in response to the Safe Drinking Water Act and by the National Institutes of Health and Centers for Disease Control and Prevention in response to the potential bioterrorist threat of these organisms. Research on microsporidiosis in the Division focuses on infection of humans and nonhuman primates by species of microsporidia, namely Enterocytozoon bieneusi and three Encephalitozoon species. Studies are being conducted to improve diagnostic tests as well as to characterize the natural history and pathogenesis of these infections. These studies serve to better understand the course of microsporidial infections in humans. Effective therapies are lacking to treat all the microsporidia that infect humans and animals, but fumagillin and related compounds show great potential. Toxicity concerns, however, have led to studies to clone, express, and characterize methionine aminopeptidase 2, the putative fumagillin target of the microsporidia, and to develop a high throughput screening assay that can be applied to identify more effective and less toxic fumagillin analogues. Tissue culture and murine and nonhuman primate models of microsporidiosis are being used for preclinical testing to evaluate efficacy and toxicity of lead compounds. In addition, immune responses are being defined in these models to better understand the mechanisms by which T cells and macrophages interact to kill microsporidia. Finally, methods are being developed and applied to capture and identify microsporidia in water sources that pose a potential risk for transmission to humans and animals. STLV and HTLVSimian and human T cell leukemia virus and (STLV and HTLV) are important pathogens causing life-long chronic infections that may lead to T-cell leukemia/lymphoma (ATLL) and a variety of neuromuscular diseases of humans. An animal model was developed in the Division using STLV which is closely related to HTLV-1. STLV was associated with abnormal lymphoproliferation and produced hyperplastic lymph nodes that were clonal outgrowths of STLVagm-infected cells similar to those seen in humans with ATLL. In addition, there was immunosuppression and widespread dissemination of a naturally occurring simian immunodeficiency virus (SIVagm). We have several species-specific isolates of STLV and are characterizing them genetically, as well as for their ability to transform lymphoid cells in vitro and for their ability to experimentally infect and cause disease in the nonhuman primate. Collaborative studies on HTLV include the experimental transmission of human clinical isolates of HTLV-I to nonhuman primates. In certain geographic regions, HTLV co-infection of HIV-I positive individuals has a prevalence of 5-10%, and is hypothesized to increase HTLV viral expression and the risk for HTLV-associated diseases. Macaques co-infected with SIV and STLV have served as a model for this human infection and disease. Varicella Virus Infection, Latency, and ReactivationThe Varicella-zoster virus causes clinically mild chickenpox in children. However, in older or immunocompromised individuals, infection is generally more severe and may be life threatening. In addition, latency of the virus in dorsal root ganglia is common, and reactivation of the virus late in life is responsible for significant morbidity due to viral zoster, or shingles. A nonhuman primate model of infection and disease in the African green monkey was developed and is being used to explore these issues. Collaborative studies on immunomodulatory therapeutics for control of infection are also underway. In additional collaborations, chimeric varicella viruses are being tested to delineate genes responsible for varicella latency, and ultimately, control of reactivation. A candidate SIV vaccine using simian varicella virus vector is under development. Respiratory Syncytial Virus Infection and PathogenesisRespiratory Syncytial virus (RSV) is a major cause of serious lower respiratory tract disease in infants and young children. Hospitalized children with underlying heart and lung conditions are at the most risk for severe complications. No effective vaccine is available, and the current antiviral drug available has limited use. A nonhuman primate model of RSV infection and disease has been developed and is being utilized to test various RSV vaccine candidates and therapeutics. RotavirusA nonhuman primate model of human rotavirus infection has been developed using a new rotavirus strain isolated from nonhuman primates at the Tulane center. The virus was characterized as serotype G3, subgroup 1, genotype P and designated the Tulane University Cincinnati (Children’s) Hospital (TUCH) strain. The rotavirus model using juvenile macaques is being used to develop new vaccines and to elucidate the mechanism(s) of how rotavirus evades the host innate response, and adaptive immunity to further characterize the correlates of rotavirus protection. RESEARCH RESOURCESThe Division of Microbiology maintains two service cores to support the clinical staff and investigators that use the resources of the TNPRC. These are: Viral Diagnostic Core (VDC) A healthy nonhuman primate (NHP) colony that is free of specific pathogens is vital to the success of a primate center. The VDC, established in 2003, is a key resource in this regard. It has two components: 1. The viral diagnostic component provides serological testing for the NHP colony. For the “Specific Pathogen Free” colony, each monkey is tested for SIV, STLV, SRV and herpes B virus infection. For SIV specific antibody detection, a peptide ELISA against SIVmac/SIVsm was developed. Confirmation of SIV antibody is provided by a Western blot (WB) assay. Herpes B virus serodiagnosis is done using a commercial Herpes simplex virus type1 ELISA. Commercial ELISA kits and a second generation WB assay that allows discrimination between STLV-1 and STLV-2 are being used for diagnosis of STLV infections. The methodology for SRV diagnosis is currently under development. The VDC processed approximately 3,000 samples in the past year and is growing to meet future needs. 2. A real-time PCR component is also part of the Viral Diagnostic Core. Currently SIVmac and SIVagm quantification is available. Other services, such as cytokine and chemokine quantification, are also available. Additionally, the Core will assist investigators with custom-designed real-time PCR assays. The VDC also provides other serological assays upon the investigator’s request. Examples are TB Primagam and SIV p27 antigenemia quantification. Retrovirus Challenge Stock Production and SIV/SHIV Isolation CoreThis Core provides research investigators with primate lentiviruses for inoculation of nonhuman primates (NHP). The Core fills this need because many investigators who use NHPs are not able to produce and maintain their own virus challenge stocks. Therefore, the Core produces and maintains titered viral stocks for testing the efficacy of candidate AIDS vaccines, for testing new anti-HIV drugs and for viral pathogenesis studies. The inventory consists of 65 separate stocks of SHIVs and SIVs. These viruses were grown in human cell lines and in primary cell cultures of human and NHP origin. The Core has also prepared specially requested stocks for use by investigators. EDUCATION & TRAINING OPPORTUNITIES (send email) Members of the Division are engaged in education and training at multiple levels including summer fellowships for undergraduates, graduate students and postdoctoral fellows. In addition, senior members of the faculty are heavily involved with mentoring junior faculty. Mentoring has resulted in new faculty obtaining significant external research support. The Division fully participates in Tulane graduate programs with Division faculty serving as major professors for these programs. STAFF (View Publications) Chair – Preston A. Marx, PhD, Professor of Tropical Medicine Faculty Didier, Elizabeth S. PhD, Professor |
|
©2013 Tulane University |
