|
  DISEASES WE INVESTIGATE DISEASES WE INVESTIGATE
Rotavirus
Overview
Rotaviruses, along with noroviruses, are the most common etiological agents of diarrhea in infants and young children worldwide. Rotaviruses have also been isolated from monkeys, domestic mammals, birds and other species.
- It has been estimated that in Asia, Africa and Latin America there are more than 1 billion cases of diarrhea each year with 2-3 million deaths. The majority of deaths occur in children less than 5 years of age. About half of all cases of severe diarrhea are caused by rotaviruses.
- Countries in southeast Asia, equatorial Africa and South America are the most affected, and approximately 600,000 deaths occur each year among children in developing countries. In the U.S., rotavirus infections result in more than 50,000 hospitalizations each year.
- Several vaccine candidates aimed at preventing rotavirus infection have been developed, but none are available for routine immunization at present.
Infectious Agent
- Rotaviruses represent a genus in the Reoviridae family. The genome contains 11 segments of double-stranded RNA that is equal to 18.5 kb. The virions are easily recognizable by electron microscopy due to their characteristic 70 nm icosahedrons that resemble a wheel (rota translates as wheel in Latin).
- Rotavirus particles are assembled in the cytoplasm, bud through the endoplasmic reticulum and, during the maturation process, acquire an outer capsid layer. Virions are non-enveloped, triple-layered, and consist of an inner core (VP1, VP2 and VP3), a middle capsid (VP6), and an outer capsid (VP7 and VP4).
- Classification of rotaviruses is based on antigenic and genetic structure derived from VP6 (groups A-E), VP7 and VP4 surface proteins. The VP7 serotype is designated as G (VP7 is glycoprotein) and VP4 serotype as P (VP4 is protease sensitive). Most human rotaviruses belong to group A. The PG4 combination is responsible for 88.5% of rotavirus diarrheas in children worldwide.
Transmission
- Rotaviruses are shed in large numbers in the feces during episodes of acute diarrhea and are transmitted by the fecal-oral route.
- Animal-to-human transmission has not been documented under natural conditions. However, human rotavirus strains that possess certain gene segments with a high degree of homology with animal rotavirus genes have been found. This was probably due to genetic reassortment between human and animal rotaviruses. Such occurrence does not appear to be of clinical importance.
- Escape of rotavirus particles beyond the gastrointestinal barrier has been reported by several groups including ours. However, the clinical significance of extraintestinal infection by rotavirus appears to be very low.
The Disease
- Rotavirus infection in its natural host occurs in the mature enterocytes at the tips of the intestinal villi. Following infection, the cells at the villus tips die and the villus becomes denuded which results in shortening and stunting of the villi. In the underlying lamina propria, mononuclear cell infiltration is observed.
- It was proposed that the clinical symptoms of vomiting and diarrhea associated with rotavirus infection are due to the loss of the mature villus enterocytes and retarded differentiation of the immature enterocytes.
- It was described that the non-structural NSP4 rotavirus protein can induce diarrhea in infant mice and therefore, it might function as an enterotoxin in humans also.
Treatment
- Mucosal and serum antibodies to rotavirus are considered to be important for protection against disease although other immune factors play an important role as well.
- Although there is no vaccine available at present, several rotavirus vaccine candidates are in final stages of clinical trials.
- Treatment of rotavirus infection is mostly symptomatic, e.g. based on attempts to reduce dehydration by application of rehydration therapy. Passive protection has also been demonstrated in humans by oral administration of gamma globulins during the first week of life or by administration of rotavirus antibodies to immunodeficient children with chronic rotavirus infections.
Our Research
- We isolated from non-human primates a new rotavirus strain that was characterized as serotype G3, subgroup 1, genotype P. Our isolate was named TUCH.
- By utilizing TUCH, we developed a macaque model of rotavirus infection. Our experimental model is characterized by the consistent and predictable pattern of rotavirus shedding in stools.
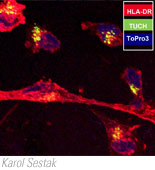
- We described CD4+/CD8+ T cell mediated Th1 and Th2 cytokine production in rhesus macaques infected with rotavirus. Cytokine production in rotavirus-infected macaques was found to resemble the pattern of cytokine production seen in rotavirus-infected humans.
- We developed novel molecular diagnostic tools that allow us to quantitatively evaluate rotavirus infection.
Future research will involve; novel vaccine candidates, pathogenicity studies to elucidate the mechanisms of how rotavirus evades the host innate response, and adaptive immunity studies to further characterize the correlates of rotavirus protection.

|
 |